Abstract
The murine double minute 2 (MDM2) oncoprotein is a key E3 ubiquitin ligase that degrades and thereby inactivates the tumor-suppressor p53. Targeting of the MDM2/p53 interaction with reversible small molecule inhibitors (SMI) to stabilize p53 and induce apoptosis in wildtype (WT) p53 tumors has been an emerging therapeutic approach in AML and in other WT p53 hematologic and solid tumor malignancies. However, recent clinical trials with MDM2 inhibitors, especially in R/R AML, have resulted in suboptimal clinical activity, highlighting the need for novel therapeutic approaches.
KT-253 is a novel, highly potent heterobifunctional MDM2 degrader that suppresses p53-dependent MDM2 protein upregulation that is known to be triggered by the MDM2 SMIs and thereby limits their clinical activity. Previously, we have shown that KT-253 has superior activity compared to MDM2 SMIs, demonstrating >200-fold improvements in both in vitro cell growth inhibition and apoptosis. Because of its superior pharmacological profile, a single dose of KT-253 was sufficient to induce rapid apoptosis and sustained tumor regression in the MV4;11 AML and RS4;11 ALL cell line-derived (CDX) mouse xenograft models, supporting an intermittent dosing schedule of KT-253.
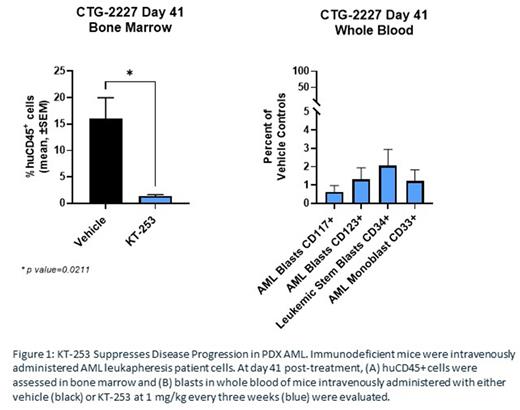
Because of the initial promising clinical activity of SMIs in AML, and the potential for KT-253 to provide superior activity, we assessed the activity of KT-253 in AML patient-derived xenograft (PDX) models. In vivo, KT-253 administered once every 3 weeks at 1 mg/kg led to tumor regression in a variety of these models, including CTG-2227 (Figures 1A and B). In addition, in an AML CDX model resistant to venetoclax such as MOLM13, KT-253 administered in combination with venetoclax once every three weeks showed more substantial activity than the single agents alone and led to sustained tumor regression.
In summary, intermittent dosing of the clinical candidate KT-253 achieved tumor regression in a variety of AML PDX and CDX models. Combination of KT-253 with the AML standard of care treatment venetoclax achieved durable tumor regression in additional AML xenograft models that are resistant to standard of care, suggesting potential benefit to an expanded patient population. In addition, we have identified hematological and solid tumor indications that respond acutely to MDM2 degradation, we plan to share more details at upcoming meetings.
Disclosures
Mayo:Kymera Therapeutics: Current equity holder in publicly-traded company. Chutake:Kymera Therapeutics: Current Employment, Current equity holder in publicly-traded company. Karnik:Kymera Therapeutics: Current Employment, Current equity holder in publicly-traded company. McDonald:Kymera Therapeutics: Current Employment, Current equity holder in publicly-traded company. Cho:Kymera Therapeutics: Current Employment, Current equity holder in publicly-traded company. Filiatrault:Kymera Therapeutics: Current Employment, Current equity holder in publicly-traded company. Chen:Kymera Therapeutics: Current Employment, Current equity holder in publicly-traded company. Dixit:Kymera Therapeutics: Current Employment, Current equity holder in publicly-traded company. Proctor:Kymera Therapeutics: Current Employment, Current equity holder in publicly-traded company. Breitkopf:Kymera Therapeutics: Current Employment, Current equity holder in publicly-traded company. Hu:Kymera Therapeutics: Current Employment, Current equity holder in publicly-traded company. Sharma:Kymera Therapeutics: Current Employment, Current equity holder in publicly-traded company. Ewesuedo:Kymera Therapeutics: Current Employment, Current equity holder in publicly-traded company. Weiss:Kymera Therapeutics: Current Employment, Current equity holder in publicly-traded company. Growney:Kymera Therapeutics: Current Employment, Current equity holder in publicly-traded company. Williams:Kymera Therapeutics: Current Employment, Current equity holder in publicly-traded company. Schalm:Kymera Therapeutics: Current Employment, Current equity holder in publicly-traded company; Blueprint Medicines: Current equity holder in publicly-traded company, Ended employment in the past 24 months.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal